COSMETICS TESTING
ZazaLab provides cosmetic testing services for manufacturers, brand owners, and contract manufacturers, supporting regulatory compliance under EU Regulation (EC) No 1223/2009. Our laboratory testing covers stability, shelf life estimation, period after opening (PAO) determination, microbiological quality, preservative efficacy, and skin compatibility, and is performed to support Product Information Files (PIF) and Cosmetic Product Safety Reports (CPSR). Additional or customized tests can be arranged upon client request, depending on product type, formulation, and regulatory needs.
Contact us now for an individual offer!
Stability of formulation
Formulation stability testing is performed during the product development phase, before the final formulation and packaging are defined. This testing is intended for evaluating multiple formulation variants in parallel, allowing comparison and selection of the most stable formulation for further development.
During testing, formulations are stored in inert glass containers and exposed to accelerated stability conditions. Over defined time intervals, key parameters such as appearance, color, odor, texture, phase behavior, and pH are monitored to identify potential instability or degradation.
The test requires 200 ml of bulk product per formulation, and the standard testing period is up to 4 weeks from receipt of samples. Results support informed formulation decisions prior to packaging selection and final stability studies.


Stability and packaging compatibility
Stability and packaging compatibility testing is performed on the final cosmetic product in its intended market packaging, as it will be placed on the market. This testing evaluates both the stability of the formulation and its interaction with the primary packaging.
The assessment includes monitoring of product appearance, color, odor, texture, phase behavior, pH, and any visible changes related to packaging interaction, such as migration, absorption, discoloration, leakage, or deformation of packaging components.
For testing, up to 15 units of the finished product in original market packaging are required. The duration of the study depends on the intended shelf life of the product and the selected testing protocol.
Product shelf-life estimation
Based on the obtained results and reports from the stability studies of formulation, stability and packaging compatibility, we provide a estimation or a justified proposal of the product’s shelf life, which serves as a key input for CPSR preparation and inclusion in the Product Information File (PIF).


Period after opening (PAO) determination
The Period After Opening (PAO) indicates the time during which a cosmetic product remains safe and stable for use after it has been opened for the first time. PAO labeling is required under EU cosmetic legislation for products with a minimum durability of more than 30 months and is expressed in months (e.g. 6M, 12M).
PAO determination is performed as a theoretical PAO estimation based on available stability data, product type, formulation characteristics, packaging system, and intended use. The assessment takes into account the results of stability and packaging compatibility testing, as well as factors influencing product exposure after opening.
The resulting theoretical PAO estimation provides documented justification for PAO labeling and supports CPSR preparation and inclusion in the Product Information File (PIF).
Microbiological testing
Microbiological testing evaluates the microbiological quality and hygienic status of cosmetic products before they are placed on the market. The purpose of this testing is to confirm that the product meets applicable microbiological criteria and does not pose a risk to consumer safety under normal conditions of use.


Challenge test
The Challenge Test, also known as the Preservative efficacy test, evaluates the ability of a cosmetic product’s preservative system to control microbial growth during its intended use. The test assesses the product’s resistance to contamination by selected microorganisms over a defined time period.
For this testing, 200 ml of bulk product is required. Test results are available approximately 1.5 – 2 months after sample receipt.
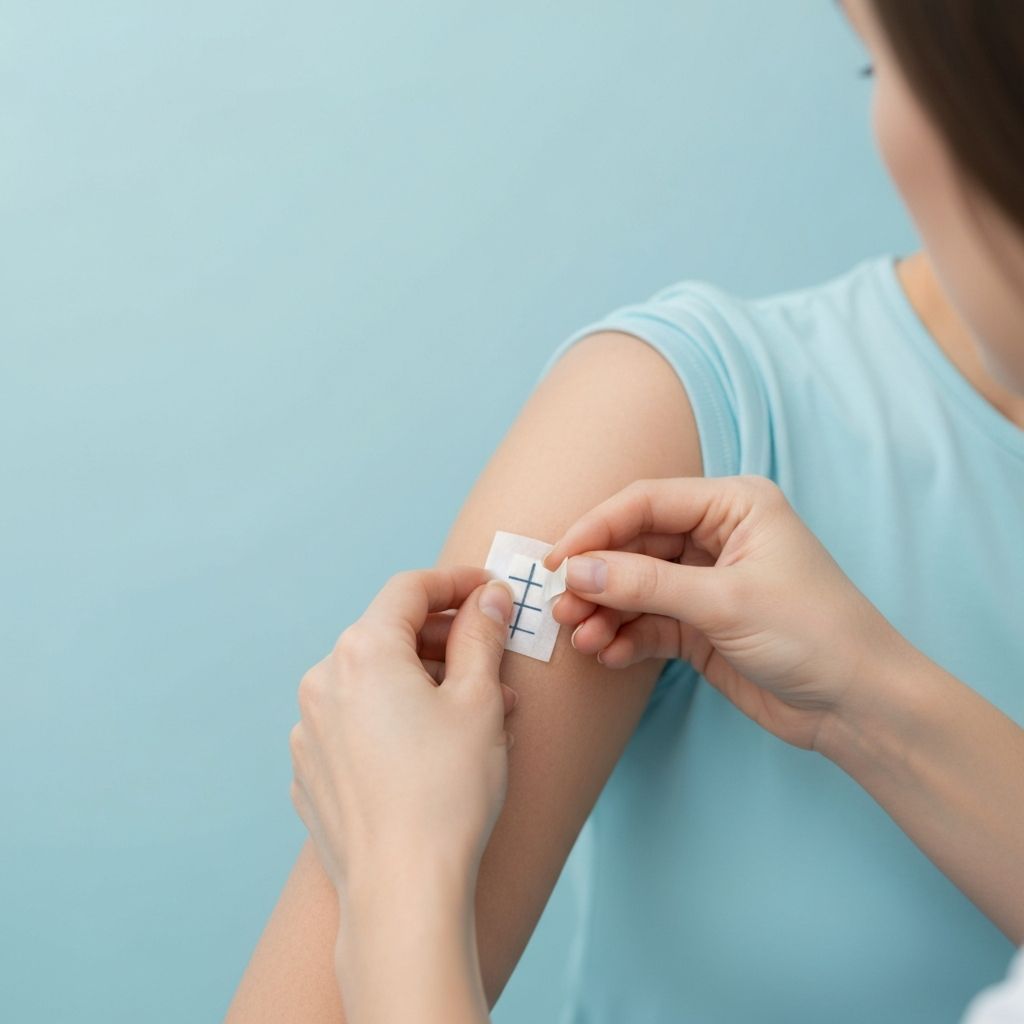
Dermatological (Patch) test
The dermatological (patch) test evaluates the skin compatibility and tolerance of a cosmetic product under controlled conditions. The test is designed to identify potential irritation reactions following topical application on human volunteers.
Patch testing provides additional safety support for cosmetic products and can be used to substantiate skin compatibility claims, where applicable. While the dermatological (patch) test contributes valuable data to the overall safety assessment, it does not replace the Cosmetic Product Safety Report (CPSR) and is used as complementary supporting documentation within the Product Information File (PIF).
For testing, 100 ml of bulk product is required. Test results are available approximately 2 months after sample receipt.